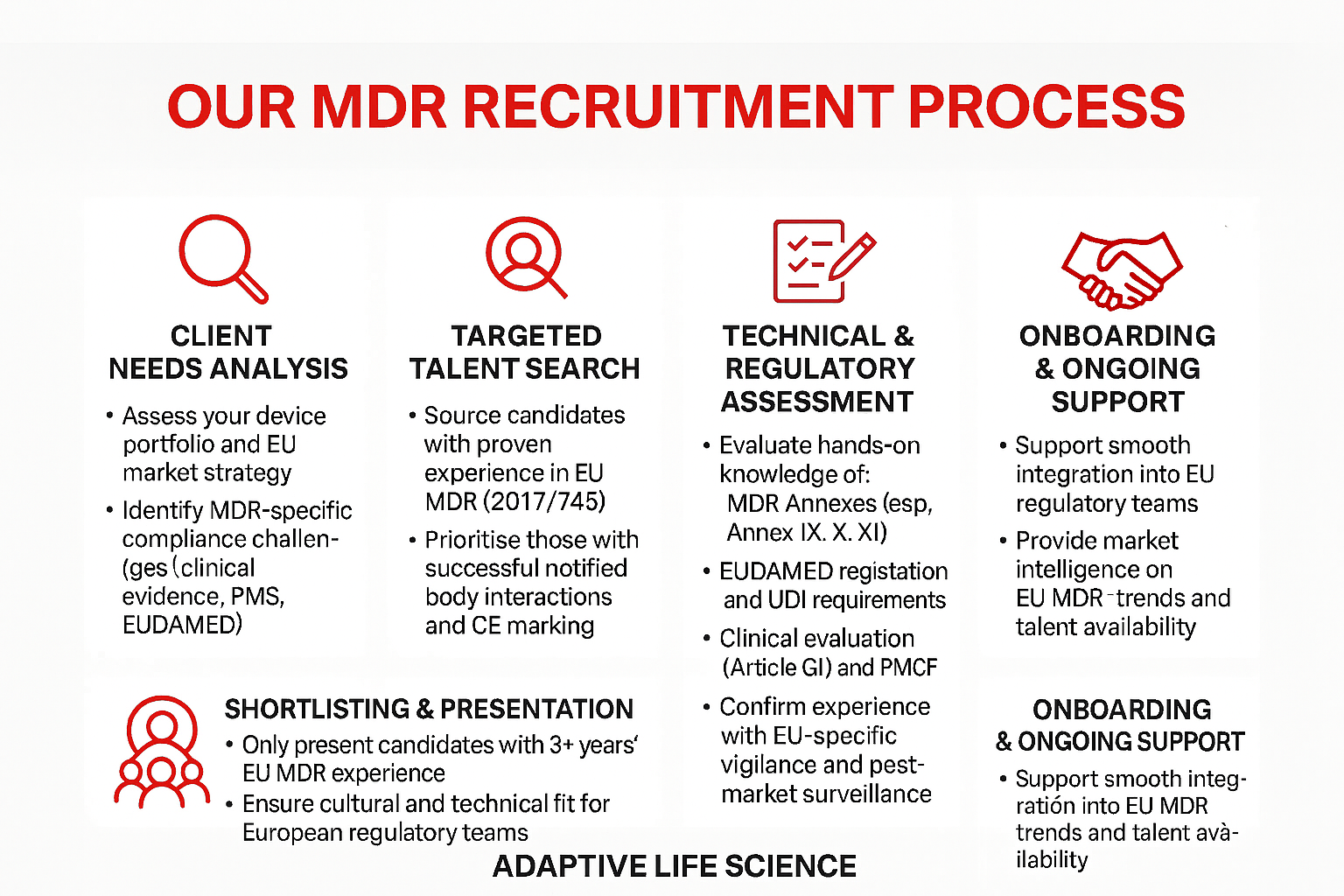
The MDR Compliance Challenge
Since the MDR's full implementation, medical device manufacturers face significantly more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. The regulation's expanded scope, enhanced scrutiny of high-risk devices, and rigorous notified body assessments have created a critical skills shortage in the market.
Companies require MDR specialists who understand not just the regulation's technical requirements, but how to implement practical compliance strategies that balance regulatory obligations with business objectives. Our recruitment expertise identifies professionals who can transform regulatory complexity into operational excellence.